Probiotic formulation granulation with rotary evaporators:
A complete guide

The challenge with probiotic granulation isn't just about drying - it's about preserving billions of living organisms while creating stable, flowable particles that survive storage and deliver their benefits intact. Every degree of temperature, every millibar of vacuum pressure, and every minute of processing time affects whether those cultures remain viable.
If you're working with probiotic formulations, you already know the stakes. A successful granulation process means the difference between a stable product with 10^9 CFU/g after six months of storage and one that loses viability before it reaches the shelf. The rotary evaporator has become the instrument of choice for this delicate balance, offering precise control over the solvent removal process that's critical for maintaining cell viability.
If you don’t want to learn about it, and you just want the rotary evaporator we built for this application, you can customize your probiotic granulation rotary evaporator package in less than 20 seconds.
Why solvent evaporation matters for probiotic granulation
Probiotic granulation typically involves wet granulation methods where bacterial cultures are suspended in a liquid binder solution containing protective excipients. The solvent - whether water, ethanol, or a mixture - must be removed without exposing the organisms to lethal temperatures or osmotic shock.
Traditional drying methods present serious limitations. Spray drying subjects probiotics to inlet temperatures of 150-200°C, even if outlet temperatures remain lower. Fluid bed drying creates mechanical stress through vigorous particle movement. Tray drying lacks uniformity and requires extended processing times that increase contamination risk.
Rotary evaporation solves these problems through controlled, low-temperature solvent removal under vacuum. The rotating flask creates a thin film of material, maximizing surface area while minimizing exposure time. Vacuum conditions allow solvents to evaporate at temperatures as low as 30-35°C - well within the survival range for most probiotic strains.
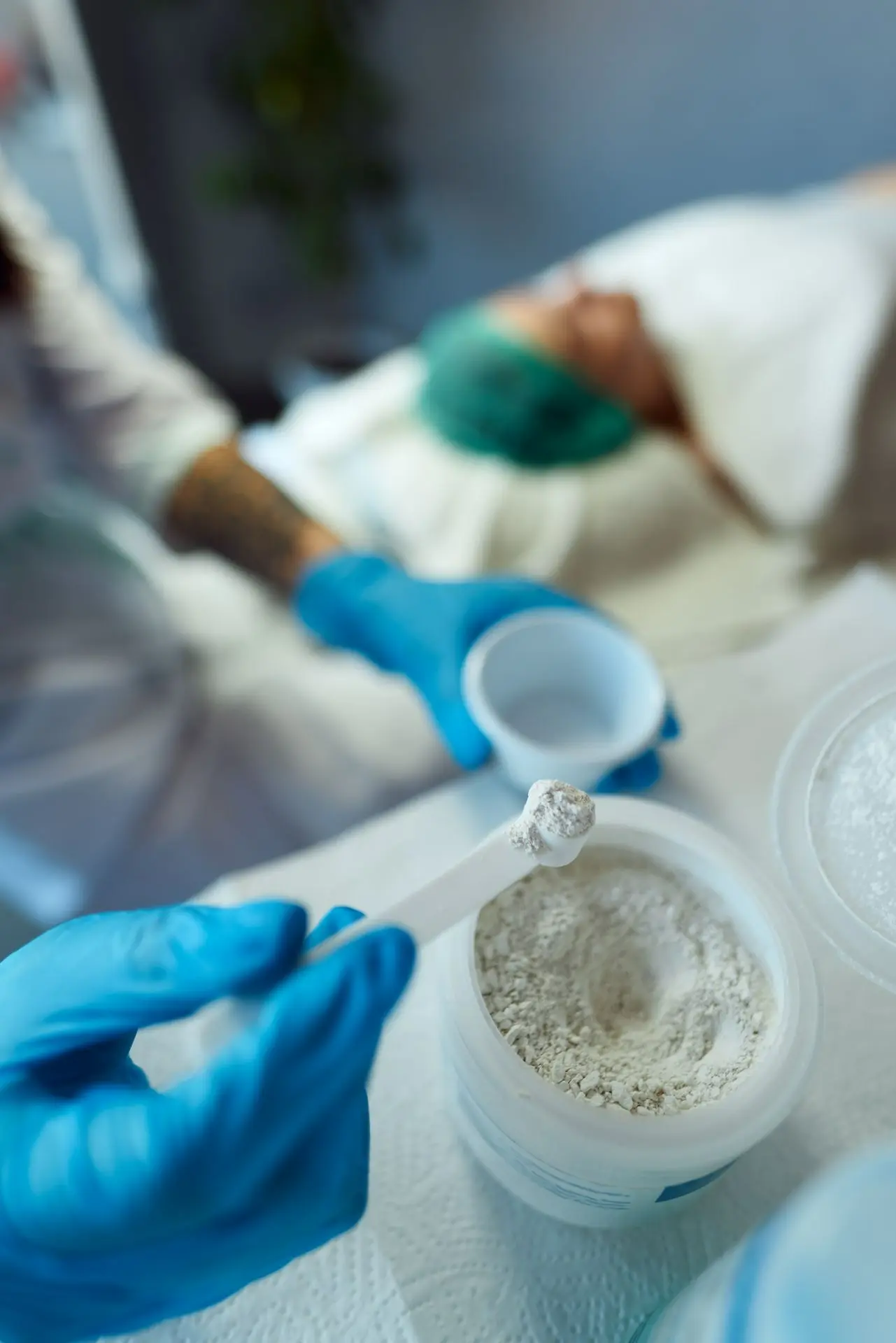
What target particle size and moisture content ensure proper encapsulation and flowability?
The target specifications for probiotic granules balance several competing demands. You need particles large enough to flow through encapsulation equipment without bridging, yet small enough to fit standard capsule sizes. You need sufficient moisture to maintain viability, but not so much that granules clump during storage.
For most probiotic formulations, aim for:
Particle size
200-600 μm for capsule filling, 100-300 μm for tablet compression
Moisture content
200-600 μm for capsule filling, 100-300 μm for tablet compression
Bulk density
200-600 μm for capsule filling, 100-300 μm for tablet compression
The rotary evaporator's contribution here is precise moisture control. Unlike batch ovens where moisture content varies by position, the rotating flask ensures uniform drying. You can monitor weight loss in real-time and stop exactly at your target moisture level. The vacuum control allows fine-tuning - lower vacuum (higher pressure) slows evaporation for better control as you approach the endpoint.
How can I maintain probiotic viability during solvent evaporation?
CFU retention during granulation depends on three factors: temperature exposure, drying rate, and protective matrix composition. The rotary evaporator addresses all three through its design.
Temperature control starts with the water bath. Most probiotic strains show minimal viability loss below 40°C, with optimal processing at 30-35°C. The vacuum allows these low temperatures to effectively remove solvents - at 50 mbar, water evaporates at 33°C, while ethanol evaporates at just 19°C. This temperature window preserves not just viability but also functionality, maintaining the organisms' ability to colonize and produce beneficial metabolites.

The drying rate matters because rapid moisture removal causes osmotic shock. Slow, controlled evaporation allows cells to adapt. Start with higher vacuum (20-30 mbar) for initial bulk solvent removal, then reduce vacuum (increase pressure to 100-150 mbar) for the final drying phase. This staged approach typically retains 85-95% of initial CFU counts compared to 60-70% with conventional drying.
Your protective matrix makes the difference between success and failure. The excipients you choose must protect during drying, storage, and eventual rehydration. Combine cryoprotectants like trehalose (5-10% w/w) with bulking agents like maltodextrin (10-20% w/w) and proteins like skim milk powder (5-15% w/w). These materials form a glassy matrix around cells during drying, preventing membrane damage and maintaining cellular integrity.
What excipients work best for probiotic granulation without harming cultures?
Not all excipients are created equal when it comes to probiotic compatibility. Some common pharmaceutical binders and fillers can actually reduce viability through pH effects, osmotic stress, or direct toxicity.
Safe and effective options include:

Protective agents
Trehalose stands out for its ability to replace water molecules around cell membranes during drying. Use it at 5-10% of dry weight. Sucrose works similarly but requires higher concentrations (15-20%). Both sugars form protective glasses at low moisture content, essentially freezing cells in a suspended state.

Bulking agents
Maltodextrin (DE 10-20) provides structure without hygroscopicity. It creates a porous matrix that allows rapid rehydration while protecting cells from oxygen. Inulin offers the dual benefit of structure and prebiotic activity, though it requires careful drying to prevent stickiness.

Protein stabilizers
Skim milk powder remains the gold standard, providing buffering capacity and membrane protection. Whey protein isolate works well for dairy-free formulations. Both should be pre-dissolved and sterile-filtered to prevent contamination.

Flow enhancers
Microcrystalline cellulose (2-5%) improves granule strength and flowability without affecting viability. Silicon dioxide (0.5-1%) prevents caking during storage but must be pharmaceutical grade to avoid heavy metal contamination.
Avoid these problematic excipients
- Calcium phosphates (alkaline pH).
- Magnesium stearate (hydrophobic barrier prevents rehydration).
- Crosslinked polymers like croscarmellose (can trap and damage cells during swelling).
What rotation speeds and vacuum settings optimize the process?
The optimal parameters depend on your flask size, fill volume, and solvent system, but general principles apply across all setups.

Rotation speed
Start at 60-80 rpm for initial mixing and film formation. This speed creates sufficient centrifugal force to spread material without causing excessive foaming. For viscous suspensions, increase to 100-120 rpm. Higher speeds (150+ rpm) risk mechanical damage to cells and can cause bumping with low-boiling solvents.
Vacuum progression
Begin with partial vacuum (200-300 mbar) to degas the suspension and prevent sudden boiling. Gradually decrease pressure over 10-15 minutes to your target vacuum. For aqueous systems at 35°C, operate at 40-60 mbar. For ethanol-water mixtures, 30-50 mbar works well. Monitor the condensation rate - if the condenser becomes overwhelmed, reduce vacuum slightly.
Fill volume
Never exceed 50% of flask capacity, ideally maintaining 30-40% fill. This ratio ensures adequate surface area for evaporation while preventing material from climbing the flask walls. For a 1L flask, process 300-400 mL of suspension at a time.
Time considerations
Most probiotic granulation runs require 2-4 hours for complete solvent removal. Rushing the process with excessive vacuum or temperature inevitably reduces viability. Plan for 60-90 minutes of active evaporation followed by 30-60 minutes of conditioning at reduced vacuum to reach target moisture.
How can I prevent rotary evaporator bumping and overheating?
Bumping - the sudden, violent boiling that sends material into the condenser - ranks as the most common problem in probiotic granulation. Beyond product loss, bumping creates aerosols that risk contamination and operator exposure.
Prevention starts with proper degassing. Before applying full vacuum, rotate the flask at atmospheric pressure for 5 minutes, then gradually reduce pressure while monitoring for bubble formation. Add boiling chips or glass beads (pre-sterilized) to provide nucleation sites, though ensure they won't damage cells through grinding action.
Temperature ramping prevents hot spots. Start your water bath 5°C below target temperature and increase gradually over 15-20 minutes. This approach allows the suspension to equilibrate and reduces the risk of sudden boiling when vacuum is applied.
The anti-foam strategy requires balance. Traditional anti-foam agents can reduce probiotic viability, but small amounts (0.01-0.05%) of food-grade silicone emulsions work without harm. Alternatively, adjust your formulation - reducing protein content or adding small amounts of ethanol (5-10%) reduces foaming tendency.
Monitor vacuum stability constantly. Fluctuations indicate either bumping onset or condenser overload. Modern rotary evaporators with programmable controls can automatically adjust vacuum in response to pressure spikes, preventing bumping before it starts.

How Yamato's programmable controls support iterative process optimization
Process development for probiotic granulation requires systematic optimization. You're balancing multiple variables - temperature, vacuum, rotation speed, and time - against competing objectives of viability retention, moisture content, and particle characteristics.
Yamato's mini rotary evaporator transforms this optimization through programmable control. Instead of manually adjusting parameters and hoping for reproducibility, you can program complete evaporation profiles with multiple stages. Create a gentle start phase (35°C, 200 mbar, 60 rpm) for degassing, transition to active evaporation (35°C, 50 mbar, 80 rpm), and finish with conditioning (30°C, 100 mbar, 40 rpm).

The programmability extends to safety features. Set maximum temperature limits to prevent accidental overheating. Program automatic vacuum release if pressure drops too rapidly, indicating potential bumping. Create pause points for sampling without breaking vacuum, allowing real-time moisture and viability testing.
Data logging capabilities mean every run becomes a learning opportunity. Track the relationship between condensation rate and product moisture. Identify the exact conditions where viability loss accelerates. Build a library of proven protocols for different probiotic strains and formulations.
The compact footprint matters more than you might think. At 330mm width, the unit fits in anaerobic chambers or biosafety cabinets, maintaining aseptic conditions throughout processing. The anti-backflow condenser prevents contamination from vacuum system backstreaming, critical when working with pure cultures.
Comparing rotary evaporation to alternative drying methods
Each drying technology has its place in probiotic processing, but rotary evaporation offers unique advantages for research-scale granulation.
Versus spray drying
Spray drying works faster and handles larger volumes, but the instantaneous heat exposure (even with inlet temperature control) typically reduces viability by 1-2 log units. Rotary evaporation maintains CFU counts within 0.5 log of starting material. Spray drying also requires 10-50L minimum batch sizes, while rotary evaporation handles 100mL to 2L batches ideal for formulation development.

Versus freeze drying
Lyophilization preserves the highest viability but takes 24-72 hours per batch and requires separate granulation steps. Freeze-dried material often lacks the density and flow properties needed for encapsulation. Rotary evaporation produces ready-to-encapsulate granules in 2-4 hours.

Versus vacuum tray drying
Tray dryers lack the agitation that ensures uniform drying. Center regions retain moisture while edges over-dry, creating batch inconsistency. The static nature also increases contamination risk during the extended drying times (8-24 hours). Rotary evaporation's continuous mixing eliminates these gradient effects.

Versus fluid bed drying
Fluid beds excel at production scale but struggle with cohesive probiotic suspensions that tend to agglomerate. The violent agitation can damage fragile strains, particularly Bifidobacterium species. Minimum batch sizes of 500g-1kg make fluid beds impractical for early development work where material is limited.
Practical troubleshooting guide
Even optimized processes encounter problems. Here's how to diagnose and fix common issues:
Problem
Viability drops below 80% retention
Check your temperature first - even 5°C above optimal can accelerate death rates. Verify vacuum gauge accuracy; incorrect readings lead to higher actual temperatures. Examine your protective matrix; inadequate trehalose or protein content leaves cells vulnerable. Consider strain-specific sensitivity; some organisms require lower temperatures (25-30°C) regardless of longer processing times.
Problem
Final moisture exceeds target
Incomplete drying usually stems from insufficient vacuum or condensation efficiency. Check condenser temperature (-40°C or lower for aqueous systems). Verify vacuum pump performance and seal integrity. Consider solvent composition; residual water in ethanol mixtures requires extended drying. Add a conditioning phase at reduced vacuum to drive off bound moisture.
Problem
Granules show poor flowability
Particle aggregation indicates excessive residual moisture or inappropriate excipients. Increase final drying time and verify moisture content analytically. Add flow enhancers like silicon dioxide post-drying. Consider your particle size distribution; excessive fines below 100 μm impair flow. A gentle sieving step post-drying can improve uniformity.
Problem
Batch-to-batch variability
Inconsistency points to manual operation problems. Implement strict SOPs for parameter ramping. Use programmable controls to eliminate operator variation. Standardize starting material preparation, especially culture concentration and excipient mixing. Log all parameters and correlate with quality attributes to identify critical control points.
Problem
Contamination despite aseptic technique
Check the anti-backflow system; standard condensers allow vacuum pump oil mist to contaminate product. Verify glassware sterilization protocols; autoclaving alone may leave endotoxins. Consider environmental factors; position the evaporator away from air currents that disturb laminar flow. Add sterile filtration steps for all excipient solutions.
Key takeaways for successful probiotic granulation
Probiotic granulation via rotary evaporation succeeds through careful attention to organism physiology and process control. The technology offers unmatched flexibility for formulation development, allowing precise optimization of conditions that preserve viability while achieving target physical properties.
Remember these critical points:
Temperature control trumps speed
maintain 30-35°C even if it doubles processing time
Protective excipients are mandatory, not optional
invest in quality trehalose and proteins
Staged vacuum protocols prevent shocking cells
gradual pressure reduction preserves viability
Programmable controls transform reproducibility
eliminate operator variability through automation
Small-scale optimization translates to production
conditions developed on 500mL batches scale predictably
The Yamato mini rotary evaporator addresses each challenge inherent in probiotic granulation. Its precise temperature control, programmable operation, and contamination prevention features make it particularly suited for developing formulations that maintain viability from lab to market. The compact design means you can maintain aseptic conditions throughout processing, while the anti-backflow condenser eliminates a common source of contamination that plagues standard rotary evaporators.
For laboratories developing next-generation probiotic formulations, the question isn't whether to use rotary evaporation - it's how to optimize the process for each unique strain and application. The controlled conditions, reproducibility, and gentle processing that rotary evaporation provides make it the logical choice for maintaining the delicate balance between creating stable granules and preserving the living organisms that give probiotics their value.


